Model-Based Systems Engineering (MBSE) is transforming the way medical device companies in the United States design, test, and deliver life-saving equipment. By offering a streamlined approach to system design, MBSE helps these companies build safer, more reliable devices that improve patient outcomes and accelerate regulatory approval processes. In this article, we’ll dive deep into MBSE’s role in the medical device industry, explaining its importance and how it benefits manufacturers, doctors, and patients alike.
What is MBSE, and Why is It Important for Medical Devices?
Model-Based Systems Engineering, or MBSE, is a modern approach to designing complex systems. Unlike traditional methods that rely on scattered documents, MBSE uses visual models and simulations to create, test, and validate designs. For medical device companies, this is critical because it allows engineers to see how every component of a device interacts with others before it is built.
Medical devices like pacemakers, ventilators, and diagnostic imaging machines are incredibly complex. They need to work seamlessly, not just mechanically, but also electronically and with software systems. MBSE helps engineers create a “blueprint” of the entire system, reducing errors and improving safety, which is vital in the healthcare industry.
Understanding MBSE in Simple Terms
Think of MBSE as a virtual playground for designing medical devices. Instead of building a prototype right away, engineers use software to create a digital version of the device. This digital model shows how every part works together, from sensors and circuits to software algorithms.
For example, if a company is designing a new insulin pump, MBSE allows engineers to test its performance in various simulated environments. They can see how it responds to different doses, battery life, and user settings—all without needing a physical prototype.
By identifying issues early in the design phase, MBSE saves time, money, and resources while reducing the risk of malfunctions in the final product.
Why Do Medical Device Companies Use MBSE?
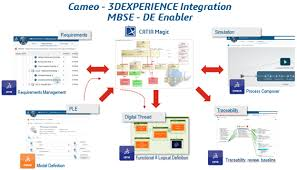
How MBSE Improves Safety in Medical Devices
Safety is the top priority for medical devices, as lives are at stake. MBSE enables companies to identify potential risks and weaknesses in a device before it reaches the market. By simulating how a device performs under different conditions, engineers can detect errors, malfunctions, or vulnerabilities that could harm patients.
For instance, a heart monitor must function flawlessly under various conditions, such as changes in temperature or electromagnetic interference. MBSE allows companies to test these scenarios virtually, ensuring the device performs safely and reliably in real-world situations.
How Medical Device Companies in the US Benefit from MBSE
In the United States, medical device companies face strict regulatory standards set by the FDA (Food and Drug Administration). MBSE makes it easier for these companies to meet these requirements by providing detailed, traceable documentation of every design decision. This speeds up the approval process and helps companies avoid costly delays.
Furthermore, MBSE improves collaboration among teams. Engineers, designers, and regulatory experts can work together in real time using shared digital models, ensuring everyone is on the same page. This reduces communication errors and helps projects move forward efficiently.
Real-Life Examples of MBSE in Action
Several leading medical device companies are already reaping the benefits of MBSE. For example:
- Medtronic, a global leader in medical technology, uses MBSE to design advanced insulin pumps and heart devices. By simulating device performance under various conditions, Medtronic ensures safety and effectiveness before production.
- GE Healthcare relies on MBSE to improve the development of its imaging systems, such as MRI and CT scanners, ensuring accurate diagnostics for patients.
- Philips Healthcare uses MBSE for its ventilators and patient monitoring systems, helping them meet strict FDA standards while delivering high-quality devices.
Benefits for Doctors and Patients
MBSE doesn’t just benefit manufacturers—it also has a direct impact on doctors and patients.
- For doctors, MBSE-designed devices are more reliable and easier to use. When a device performs consistently, doctors can trust it to deliver accurate results, whether it’s a surgical tool or a diagnostic machine.
- For patients, safer devices mean fewer risks and better outcomes. Whether it’s a life-saving implant or a diagnostic tool, MBSE ensures that medical devices meet the highest safety and quality standards.
MBSE and Faster Medical Approvals
The path from concept to market for medical devices can be long and challenging. Regulatory bodies like the FDA require extensive documentation, testing, and validation to ensure patient safety. MBSE helps streamline this process by creating a digital trail of every design decision, test, and simulation.
Instead of submitting stacks of paperwork, companies can provide digital models that clearly show how the device was developed and tested. This transparency speeds up the approval process, allowing life-saving devices to reach patients faster.
The Role of MBSE in Designing Medical Equipment
Medical equipment like ventilators, surgical robots, and diagnostic tools require precision and reliability. MBSE plays a crucial role in the design process by enabling engineers to:

- Create detailed models that show how each part of the device works.
- Simulate real-world conditions to identify potential issues early.
- Collaborate across teams, ensuring that mechanical, electrical, and software components work together seamlessly.
How MBSE Helps in Testing Medical Devices
Testing is a critical phase in medical device development. MBSE allows companies to run virtual tests on their devices before building physical prototypes. For example, engineers can simulate how a blood pressure monitor performs under different conditions, such as varying cuff sizes, patient ages, and health conditions.
This approach reduces the need for multiple physical prototypes, saving time and money. It also ensures that the final device is thoroughly tested and reliable.
Why MBSE is Better Than Traditional Methods
Traditional design methods rely heavily on documents, spreadsheets, and physical prototypes. These methods can be time-consuming, error-prone, and difficult to manage, especially for complex devices.
MBSE eliminates these challenges by creating a centralized, visual model of the device. This not only improves accuracy but also allows teams to work more efficiently. With MBSE, companies can identify and solve problems early, reducing the risk of costly delays and recalls.
Challenges and Solutions for MBSE in the Medical Field
While MBSE offers many advantages, it’s not without challenges. Some common issues include:
- High initial costs: Implementing MBSE requires investment in software, training, and resources. However, these costs are often offset by long-term savings.
- Resistance to change: Some companies may be hesitant to adopt new methods. Providing training and highlighting MBSE’s benefits can help overcome this resistance.
- Complexity: For very large projects, MBSE models can become complex. Breaking models into smaller, manageable components can simplify the process.
Top Medical Device Companies Using MBSE
Many leading medical device companies in the United States are adopting MBSE to improve their products. Companies like Medtronic, GE Healthcare, Philips Healthcare, and Boston Scientific are leveraging MBSE to design safer, more reliable devices that meet regulatory standards and improve patient outcomes.
These companies are setting an example for the rest of the industry, proving that MBSE is the future of medical device development.
The Bottom Line
MBSE is revolutionizing the medical device industry by providing a modern, efficient way to design, test, and deliver life-saving equipment. For medical device companies in the United States, it’s more than just a tool—it’s a competitive advantage. By improving safety, reducing errors, and accelerating approvals, MBSE ensures that doctors and patients have access to reliable, high-quality devices.
As more companies embrace MBSE, the future of medical device development looks brighter than ever. Whether it’s designing a new surgical robot or improving diagnostic tools, MBSE is paving the way for safer, more effective medical technology.











Leave a Reply